The Science Behind UV Resistance of PVC Products
Written by Christopher G. Robertson, PhD
Polymer Technology Services LLC, Akron, Ohio, USA
Polyvinyl chloride (PVC) is one of the most versatile and widely used thermoplastic polymers. From construction materials like roofing, vinyl siding, and window frames (Figure 1) to outdoor fencing, decking, and furniture to billboards and signs, PVC's durability, weather resistance, and cost-effectiveness make it a popular choice in outdoor applications. However, like all polymers, PVC is susceptible to degradation by environmental factors, especially ultraviolet (UV) radiation from the sun. While PVC exhibits a relatively high resistance to many environmental conditions, prolonged exposure to UV light without protection can lead to significant deterioration, both aesthetically and structurally. To ensure longevity and performance in outdoor settings, it is necessary to understand and enhance PVC's UV resistance. This brief article gives an overview of the science behind UV degradation and the strategies used to improve the resistance of PVC against it.
Figure 1.
Outdoor product examples of PVC used in vinyl siding and window framing material for building construction. Image was AI generated using ChatGPT on April 5, 2025.
What Causes UV Damage in PVC?
Intro to photodegradation and how UV affects polymers
Degradation of polymers can be initiated by energy from heat, light, or mechanical sources. Light-induced deterioration of polymers is called photodegradation. The intensity of light energy is quantified by the product of Planck’s constant (h) and the frequency (ν) of the electromagnetic wave, the latter inversely related to wavelength. Compared to visible light, UV radiation has higher frequency (lower wavelength) and hence higher energy (hv) to potentially initiate the degradation process of polymers. The UV rays of relevance to outdoor exposure include the wavelength range from 315 to 400 nm known as ultraviolet A (UVA) which makes up 95% of the UV radiation at the Earth’s surface. Ultraviolet B (UVB) has wavelengths from 280 to 315 nm and accounts for the remaining 5% of UV present at ground atmosphere. The UVB radiation has higher energy than UVA, so UVB is potentially more damaging although it occurs at a significantly lower concentration.[1]
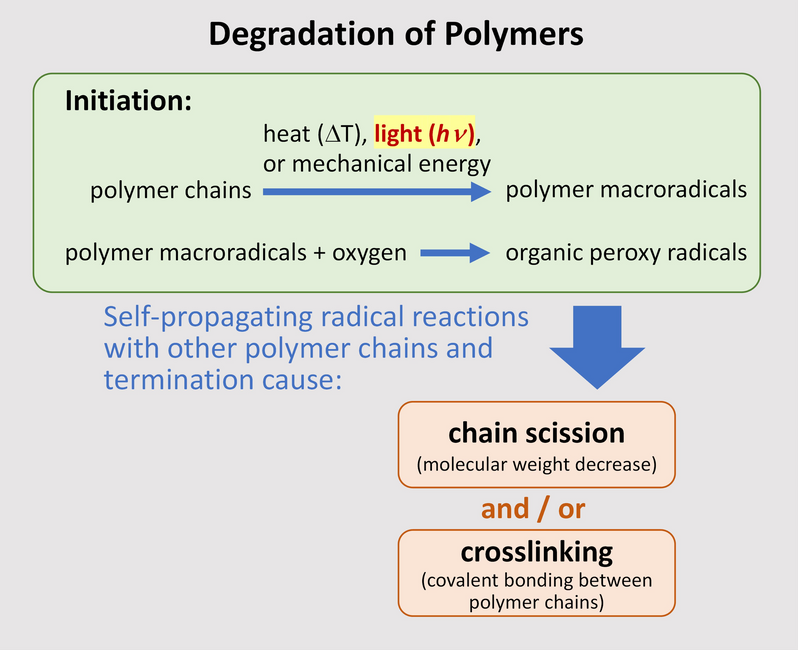
The UV light energy absorbed by the surface of a polymer material can initiate the degradation process by breaking chemical bonds in the polymer backbone or side groups which results in the formation of polymer macroradicals. These macroradicals can additionally react with oxygen present – that diffused into the material from the air – to form reactive organic peroxy radicals. The macroradicals and peroxy radicals formed in this initiation phase then propagate a free radical chain reaction with other polymer chains that, upon termination, ultimately leads to two possible changes to the polymer structure: (1) chain scission, which is a reduction in molecular weight (shorter chain length) compared to the original polymer molecular weight; and/or (2) crosslinking, which is covalent linking of polymer chains together to form a network. This process is schematically shown in Figure 2, which is a simplified outline of polymer degradation. Whether chain scission or crosslinking is more prevalent depends on a number of factors, especially the polymer type. For photodegradation of PVC, the principal outcome is chain scission which is more severe near the surfaces of the UV-exposed material.[2]
Figure 2.
Simplified schematic of the degradation process for polymers.
In many polymers, including PVC, photodegradation results in changes in color, reduced mechanical strength, and loss of flexibility. Considering specifics for PVC beyond the general picture of polymer photodegradation given above, the carbon-chlorine bonds in PVC are susceptible to breaking in the photodegradation process, which in turn creates conjugated double bonds in the polymer chain. Introduction of these double bonds into the structure during UV exposure causes PVC to change color toward a yellowish or brownish hue. This discoloration is not only unattractive but also an indicator of the parallel deterioration in polymer structure and mechanical properties.[3]
How Titanium Dioxide (TiO₂) Protects PVC
Functions by Absorption and Scattering Effects
One of the most common and effective strategies for improving UV resistance in PVC is the incorporation of titanium dioxide (TiO₂), a white particulate filler/pigment that acts as a UV blocker when compounded into a PVC formulation. TiO₂ commonly exists in two crystalline forms which are rutile and anatase, with rutile being more photostable and preferred for outdoor applications. The TiO₂ protects PVC from UV damage in two ways. TiO₂ absorbs harmful UV radiation before it can reach the PVC matrix, and TiO₂ scatters both UV and visible light thus reducing the light energy that penetrates into the PVC. Both absorption and scattering functions of TiO₂ work on preventing the initiation phase of photodegradation discussed earlier by reducing the light energy exposed to the polymer.
Addition of TiO₂ to a PVC formulation significantly delays and limits the degree of UV-induced chain scission, discoloration, and mechanical breakdown of PVC.[2,4] An example is given in Figure 3 which shows results from a scientific study by Andrady and coauthors.[5] Incorporating 5 wt% TiO₂ in a PVC compound reduced the initial yellowness index of PVC from 21.8 to 5.2 (see dashed lines in the figure) due to the bright white nature of this pigment. Addition of the 5 wt% TiO₂ provided UV resistance to the PVC which was evident from substantially less color change (less yellowing) upon exposure to light in the UVA and UVB ranges compared to PVC without TiO₂ (see yellow shaded areas in the figure).
Figure 3:
Yellowness index (measured using colorimeter) of extruded PVC, with and without addition of 5 wt% of TiO₂, after exposure to monochromatic light of the indicated wavelengths at a temperature of 23 °C. The dashed lines represent the yellowness values of the initial materials (no light exposure). The yellow shaded areas indicate the color change due to light exposure. The figure was created by plotting tabulated data provided by Andrady et al.,[5] and additional experimental details can be found in that reference.
New Innovation from Vecor Technologies
TiO₂ Extender for More Cost-effective PVC Formulation
The amount of TiO₂ used in PVC outdoor products can vary from about 2 to 10 wt% depending on the specific application, with 4 to 6 wt% being a common range. A recently introduced material innovation from Vecor Technologies is a processed aluminosilicate powder blend named VC-PVCW that can be used as a TiO₂ extender to reduce the loading of TiO₂ by 30 to 40% while maintaining UV resistance and other performance characteristics. The use of VC-PVCW enables a more cost-effective PVC formulation as this material costs about 20 to 30% less than TiO₂. This fine powder was partially developed from industrial waste stream minerals, so VC-PVCW also provides increased sustainability and circularity to PVC compounds.[6]
Other UV Stabilizers for PVC Durability
Includes Organic UV Absorbers, HALS, and Antioxidants
Additives known as organic UV absorbers can supplement the UV absorption and scattering protection from TiO₂. The TiO₂ particles are fine (diameter < 0.5 micrometer) and can be dispersed well within the PVC during compounding, but the particles are not intimately mixed with the polymer chains at a molecular level. Organic UV absorbers are miscible with the PVC chains and provide extra fortification against photodegradation from UV light that is not deterred by the TiO₂ and makes it into the PVC matrix. In PVC outdoor products, the most widely used classes of organic UV absorbers that are compounded into the formulation are benzotriazoles and benzophenones. These additives function by absorbing harmful UV radiation and dissipating it as low-level heat, and they are typically used in combination with TiO₂. Benzotriazoles are favored in modern PVC compounds for their high UV absorption efficiency, good thermal stability, and low volatility. Benzophenones, while still used in some commercial formulations, are older technology and generally offer less stability and narrower UV absorption.[2]
Carbon black, which is a particulate reinforcing filler for plastics and rubber, is an effective material for enhancing the UV resistance of PVC and other polymers. Carbon black absorbs UV radiation and converts it to heat, but its black color limits its use to dark-colored PVC applications. Also, the heat generated by absorption of UV and visible light can be a negative attribute for certain outdoor products and can possibly lead to thermal energy-initiated degradation (heat aging) if severe enough.
Preventing initiation of the photodegradation process – by using TiO₂ and optionally a complementary organic UV absorber – is key to imparting UV resistance to PVC. However, it is not possible to completely prevent all UV rays from reaching the polymer chains across the desired lifetime of an outdoor product, so some radicals will invariably be initiated. A supplementary approach is therefore to use other additives to act in scavenging/neutralizing any radicals that are formed, which addresses the radical propagation component of degradation. Hindered phenolic antioxidants and phosphite antioxidants are commonly used radical scavengers in PVC applications due to their proven performance, compatibility with PVC processing, and synergy with UV absorbers. Organotin type stabilizers are used in PVC mainly for protecting against heat aging in some products, but they can also offer stabilization against early-stage radical formation from UV radiation. A highly effective type of UV stabilizer used in diverse polymer products is the class of hindered amine light stabilizers (HALS). HALS trap and neutralize free radicals, and HALS are regenerative, meaning they can continuously cycle through their stabilizing function. The use of HALS in PVC compounds is not straightforward, however, as PVC degradation involves the release of hydrogen chloride (HCl) which can deactivate HALS or hinder their performance, especially compared to how well HALS work in polyolefin plastics like polypropylene and polyethylene. For HALS to sufficiently perform in PVC, formulators often need to include acid scavengers or co-stabilizers to neutralize HCl and maintain HALS activity.[2]
The success of PVC in a wide array of outdoor products depends on its UV resistance. Without additives, PVC is vulnerable to UV photodegradation that manifests as yellowing and loss of mechanical properties. Fortunately, there are existing options for significantly improving PVC’s UV resistance such as adding TiO₂ pigment (which both absorbs and scatters UV light), TiO₂ extender VC-PVCW from Vecor Technologies, organic UV absorbers, and antioxidants/stabilizers to neutralize free radicals. As demand for outdoor PVC products continues to grow, so too will innovations in UV stabilization technologies.
References
1. https://en.wikipedia.org/wiki/Ultraviolet; accessed April 11, 2025.
2. G. Wypych, PVC Degradation and Stabilization, 4th Edition, ChemTec Publishing, Toronto, Ontario, Canada (2020).
3. L.G. Close, R.D. Gilbert, and R.E. Fornes, Poly(Vinyl Chloride) Degradation — A Review, Polym.-Plast. Technol. Eng. 8, 177–198 (1977). https://doi.org/10.1080/03602557708545035.
4. T.-C. Yang, T. Noguchi, M. Isshiki, and J.-H. Wu, Effect of titanium dioxide on chemical and molecular changes in PVC sidings during QUV accelerated weathering, Polym. Degrad. Stab. 104, 33-39 (2014). https://doi.org/10.1016/j.polymdegradstab.2014.03.023.
5. A.L. Andrady, A. Torikai, and K. Fueki, Photodegradation of rigid PVC formulations. I. Wavelength sensitivity to light-induced yellowing by monochromatic light, J. Appl. Polym. Sci. 37, 935–946 (1989). https://doi.org/10.1002/app.1989.070370408.
6. https://www.vecortech.com/vc-pvcw-information-and-tds; accessed April 11, 2025.